What Is Required On A Prescription Label
The serial number of the prescription. For example the label might read.
 Prescriptions And Refills Facey Medical Group
Prescriptions And Refills Facey Medical Group
It uniquely identifies all drug products sold in a dosage form in canada and is located on the label of prescription and over the counter drug products that have been evaluated and authorized for sale in canada.
What is required on a prescription label. Legal requirements of what must be on a prescription label. General requirements for prescription drug labeling. Requirements on the content and format of labeling for human prescription drug.
The name and address of the pharmacy. The pharmacy then produces a label that goes on the medication pack or bottle dispensed. Your prescription label should have the drug.
Repackaging occurs when a drug product is removed from its original manufactured packaging to be placed in new packaging. What information is required to be on the prescription label. The next type of information on your prescription label is related to the drug itself.
Veterinary prescription drugs are those drugs restricted by federal law to use by or on the order of a licensed veterinarian section 503f food drug and cosmetic act. Only enough medication as needed for a limited time period should be repackaged. Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Prescriptions are medications dispensed to a patient upon submitting a valid prescription order. Frequently asked questions for pharmacists on information required on prescription labels. The law requires that the drug sponsor label such drugs with the statement.
Federal law prohibits the transfer of this drug to any person other than the patient for whom it was prescribed 1 in general prescriptions for. Name and address of the dispensing pharmacy. The prescription label for controlled drugs in addition to the above must comply with the label requirements of the federal and state uniform controlled substances act including the transfer warning auxiliary label.
Required information on labels and prescriptions. The following information must be on every prescription label. Serial number of the prescription.
Labels for schedule ii to iv medications are also required by the us food and drug administration fda to contain the following statement. The quality of the labeling is extremely important to the patients perception of the quality. Date of the prescription.
30 tab lisinopril 10mg which would mean you are supposed to have gotten 30 tablets of lisinopril 10mg. The label must comply with state and federal regulations and should correctly and clearly convey all necessary information regarding dosage mode of administration and proper storage of the product. Improved fda prescription drug labeling.
Blank Prescription Label Template Medication Medicine Bottle Pluggedn
Inspirational Medication Label Stickers Acilmalumat
Drug Prescription Template Medication List Form Template Medical
 Understanding Prescription Medication Labels Rx Outreach
Understanding Prescription Medication Labels Rx Outreach
Labels Per Sheet Template Word Community Document Template Avery 14
 Understanding The Data Required To Meet The Pllr Sciformix
Understanding The Data Required To Meet The Pllr Sciformix
 Hydrocodone Pills And Prescription Bottles With Non Proprietary
Hydrocodone Pills And Prescription Bottles With Non Proprietary
 9 Chapter 6 Medication Order Entry And Fill Process
9 Chapter 6 Medication Order Entry And Fill Process
 How To Read A Prescription Label Union Health Center
How To Read A Prescription Label Union Health Center
 Amazon Com Medvalue Medication Labels 2 1 4 X 7 8 Health
Amazon Com Medvalue Medication Labels 2 1 4 X 7 8 Health
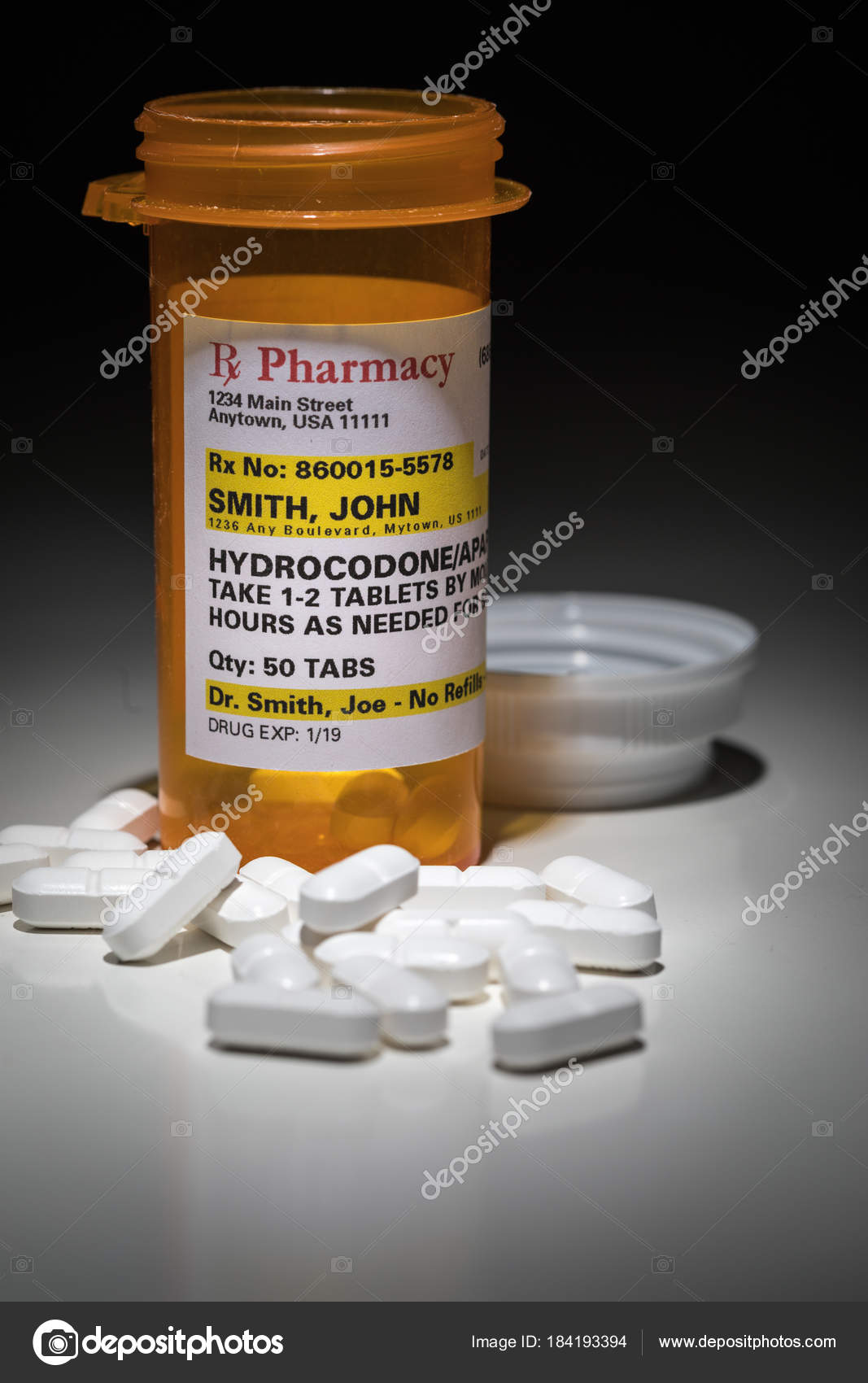 Hydrocodone Pills And Prescription Bottle With Non Proprietary Label
Hydrocodone Pills And Prescription Bottle With Non Proprietary Label
Romana Pharmacy Ltc Rh Packaging And Labeling
 Prescription Medicines And Your Child
Prescription Medicines And Your Child
 Liquid Medication Errors And Dosing Tools A Randomized Controlled
Liquid Medication Errors And Dosing Tools A Randomized Controlled
 Understanding Unapproved Use Of Approved Drugs Off Label
Understanding Unapproved Use Of Approved Drugs Off Label
 Pharmacy And Medication Information System Phis Health Care
Pharmacy And Medication Information System Phis Health Care
Prescription Bottle Label Template Drug List Entrerocks Co
 Prescription Bottle Label Template Walgreens Rx Label Simple Rx
Prescription Bottle Label Template Walgreens Rx Label Simple Rx
 Good Label And Package Practices Guide For Non Prescription Drugs
Good Label And Package Practices Guide For Non Prescription Drugs
 Hydrocodone Pills And Prescription Bottle With Non Proprietary Label
Hydrocodone Pills And Prescription Bottle With Non Proprietary Label
 Module 6 Medication Managemen Simplebooklet Com
Module 6 Medication Managemen Simplebooklet Com
 Login To The Mcguff Family Of Companies
Login To The Mcguff Family Of Companies
 How To Read A Medication Label Youtube
How To Read A Medication Label Youtube
3 Steps To Hassle Free Rx Refills And Renewals
Drug Labels Farad S Species Pages
 Biologics Rules Pregnancy And Lactation Labeling Final Rule
Biologics Rules Pregnancy And Lactation Labeling Final Rule
 Information Required On A Medication Label 1507302324017 Top Label
Information Required On A Medication Label 1507302324017 Top Label
 Prescription Refills Super Thrifty Drugs
Prescription Refills Super Thrifty Drugs
Medication Label Template Rx Pharmacy Lupark Co



malegra gold 100mg is used to treat male sexual function problems . In combination with sexual stimulation, sildenafil works by increasing blood flow to the penis to help a man get and keep an erection.
ReplyDelete